Data and beyond
Data management services and EDC platform for all types of clinical trials.
- We provide Data Management services
- User focused, flexible and robust EDC platform
- We take care of everything, including EDC setup and hosting
- All at competitive prices
Rigorous, solid, comprehensive
Clinical Data Management Services
Data Management services
We provide comprehensive Data Management and eCRF design services to the pharmaceutical industry, CROs and academic research organizations.
We assist you with studies of any size, from small Phase I studies to large-scale trials involving sophisticated designs and long-term follow-up across clinical settings and geographies.
We work in collaboration with your preferred CRO or one of our partner CROs to combine the full range of services needed for a successful execution of your study.
- Clinical database creation and validation
- Continuous data cleaning and QC
- Trial master file management
- Development of custom status reports

Outstanding, powerful, easy to use
Rodano EDC technology
Rodano EDC platform
RodanoTech provides a modern, fully featured and compliant EDC solution. Users can focus on their work and you can save the costs.
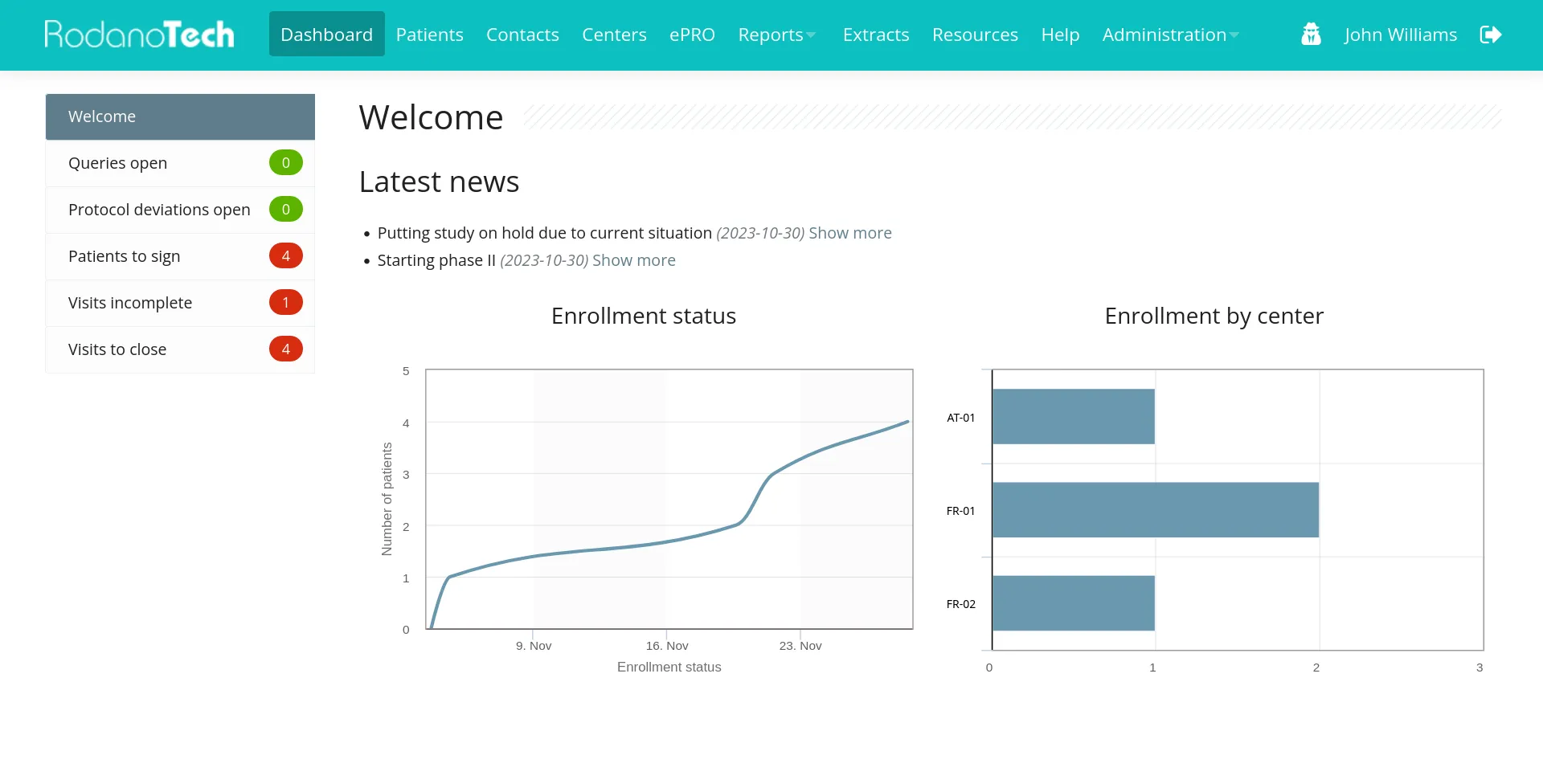
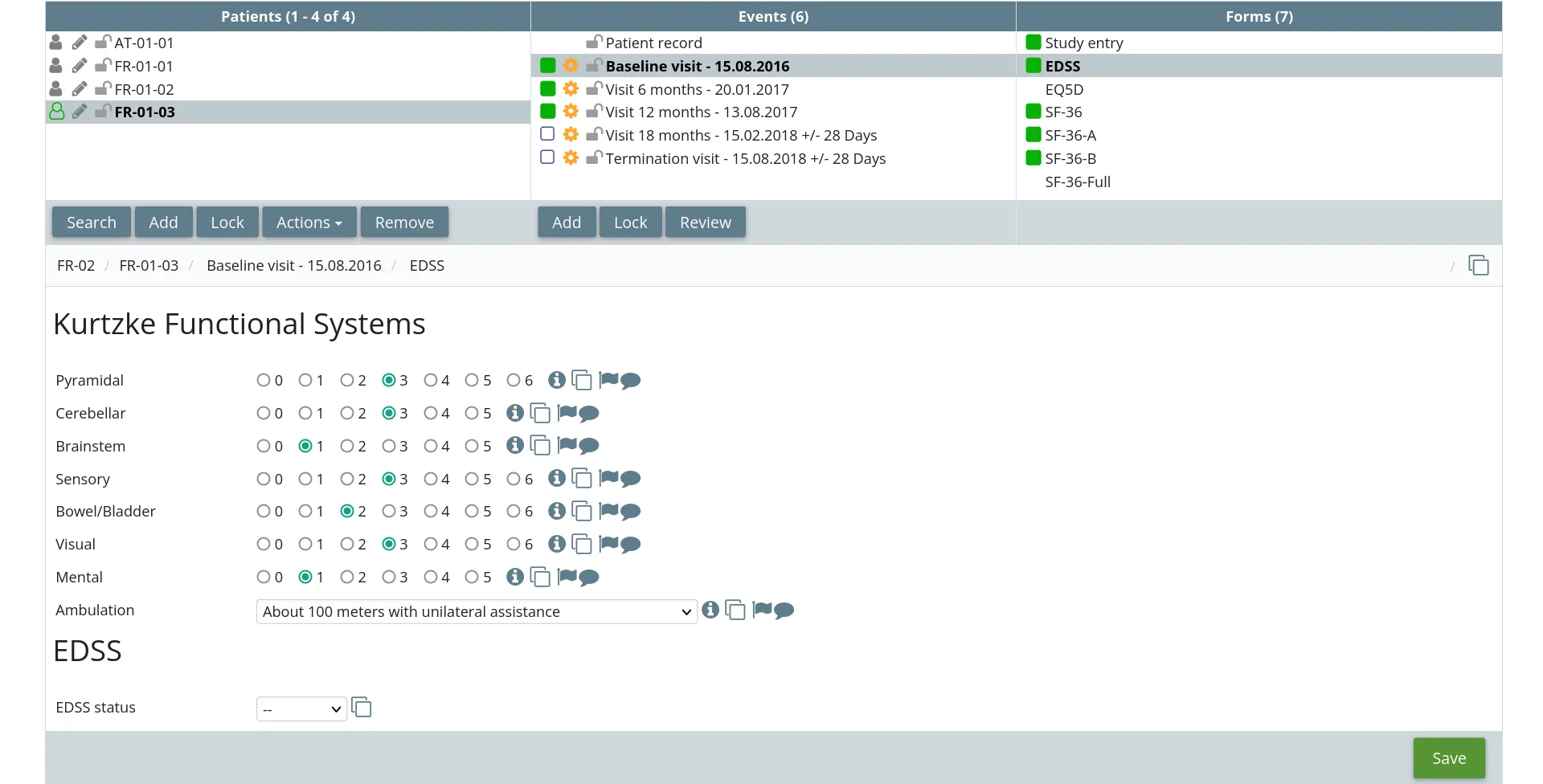
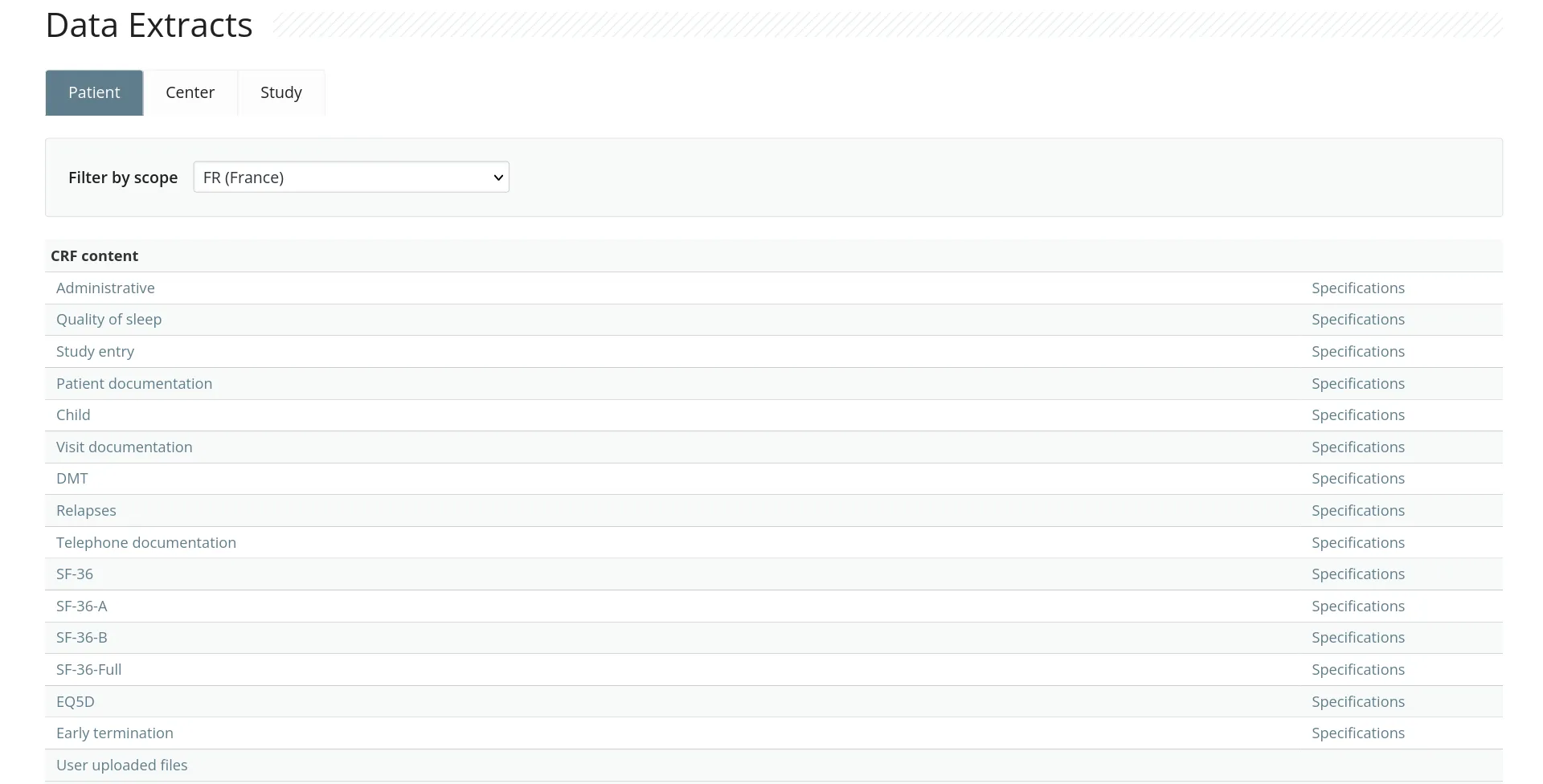
Our battle-tested EDC can be configured to handle any study. Featuring a modular design, our EDC can be configured to display custom graphs and widgets, execute custom validation rules, collect patient data with the ePRO application, classify users with custom profiles and much more.
- Modern, user-friendly and secure
- Compliant with all the standards: 21 CFR Part 11, ICH GCP, GDPR
- Very short setup time
- The most flexible configuration on the market
- Optional modules on demand: ePRO, extensible reporting tools, validation, patient graphs and more
Happy clients













About us
RodanoTech is a Swiss-based CRO with more than 15 years of experience in Data Management services and EDC solutions.
We provide quality services and develop our own custom solutions to complex problems.
Our mission is to combine our creativity, experience and technology to provide services and products that simplify life for our clients and deliver ultimate satisfaction.
Some numbers
folder_shared
100K
Patient records managed
monitoring
+50
Studies powered by Rodano EDC
home
+1000
Sites using Rodano EDC
Contact us
mail
info@rodanotech.ch
location_on
Campus Biotech Innovation Park
Avenue de Sécheron 15
1202 Geneva, Switzerland
